Notes on the import of Chinese herbal medicines
Types of “Chinese herbal medicines”: Chinese herbal medicines are generally categorized into two main types, including plant origin and animal origin. Plant-derived Chinese herbal medicines generally refer to the roots, stems, leaves, flowers, fruits, etc. of all kinds of plants, while animal-derived Chinese herbal medicines are mostly the dried carcasses of the whole animals.
The process of importing Chinese herbal medicines-
With the acceleration of the internationalization of Chinese medicine and the growth of domestic demand for Chinese herbal medicines, the market scale of imported Chinese herbal medicines is expanding.
1. Import of source standards: Chinese herbal medicines must comply with the “Import Access Conditions” issued by the General Administration of Customs, e.g.: Sand nuts (Laos, Myanmar), Cardamom (Myanmar), Comfrey (Pakistan), Dillon (Thailand), Dried Seahorses (Thailand) and so on.

Regions with overseas enterprise registration lists must meet the requirements of enterprise registration lists issued by the General Administration of Customs.
2. Domestic consignee qualification: Domestic consignee enterprises shall have the qualification of pharmaceutical enterprises such as “Pharmaceutical Business License” or “Pharmaceutical Manufacturing License

3. “Certificate of Approval for Imported Medicinal Materials” and “Customs Clearance for Medicines”: According to the announcement of “Measures for the Administration of Imported Medicinal Materials” issued by the State Food and Drug Administration, the General Administration of Customs and the Market Supervision Bureau, the “first time importer of medicinal materials” is required to apply for the “Certificate of Approval for Imported Medicinal Materials” beforehand. According to the State Food and Drug Administration, General Administration of Customs, Market Supervision Bureau issued the “Management of Imported Medicinal Materials” Notice, “the first imported medicinal materials” need to apply for “imported medicinal materials approval certificate” in advance
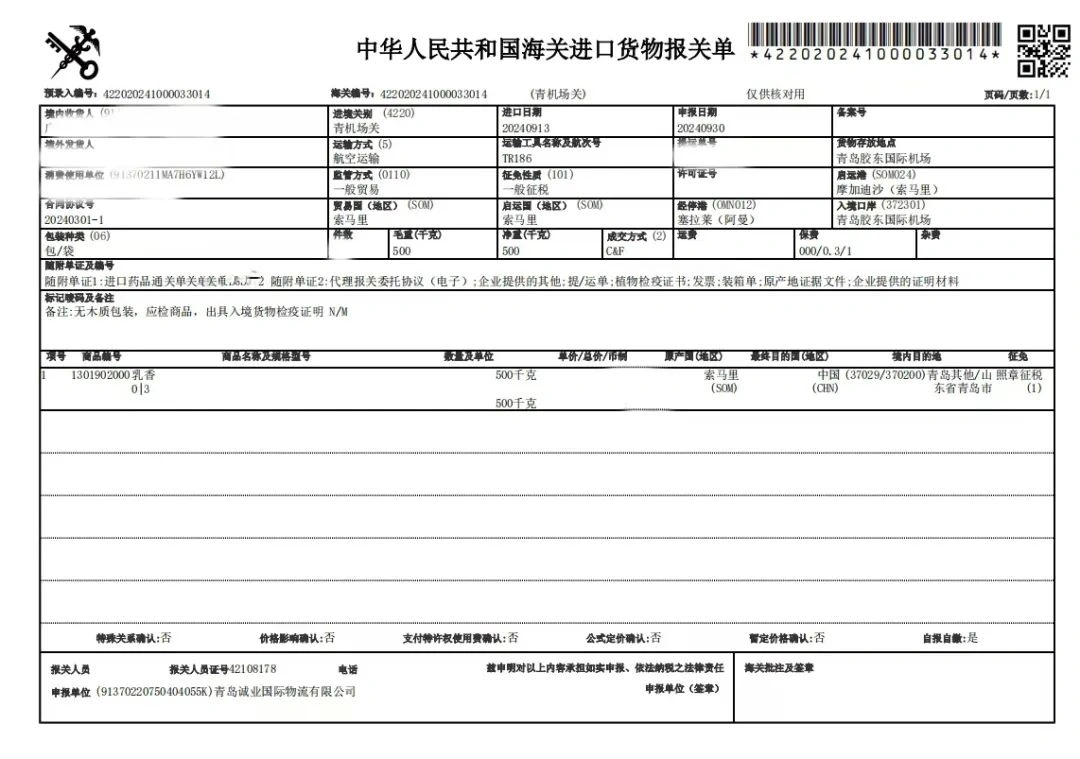
4. Import declaration materials include: certificate of origin, health certificate (animal), phytosanitary certificate (plant), trade contract, invoice, packing list, bill of lading, cargo label, imported animal and plant quarantine permit, drug clearance, endangered species certificates (to see whether the product is an endangered species). When applying for the “Entry Animal and Plant Quarantine License”, it is necessary to submit the storage agreement, so it is necessary to sign such agreement in advance with the enterprises designated for the storage and processing of Chinese herbal medicines, or apply for filing in advance.
5. Drug Inspection: According to the “Management Measures for Imported Medicinal Materials”, imported Chinese herbal medicines in the fill in the declaration, need to be imported at the port of acceptance of the inspection for the “Drug Customs Clearance”. Qualified by the port inspection of imported herbs can be sold and used. (Port drug testing organizations should generally be sampled within 20 days after the completion of the test, and issued a test report of imported herbs)
6. Market regulation: the packaging of imported herbs must meet the quality requirements of imported herbs, to facilitate storage and transportation and import inspection. Each package must indicate the Chinese name of the herbs, batch number (except for non-first imported herbs), origin, marking number, the name of the importing unit, the name of the exporter, the port of arrival, weight and processing and packaging date. Qualified products will be subject to the supervision and management of the Market Supervision Bureau in the subsequent sales process, and invoicing records are required in the normal circulation process.
Customs on how to supervise the import of Chinese herbal medicines
1. Quarantine access
The General Administration of Customs implements a quarantine access system for imported Chinese herbal medicines, including product risk analysis, evaluation and review of the regulatory system, determination of quarantine requirements, registration of overseas production enterprises and entry quarantine.
2、Enterprise Management
Domestic shippers or their agents management: should establish a record system for the import and sale of Chinese herbal medicines, processing, make relevant records and keep them for at least 2 years. At the same time should be equipped with Chinese herbal medicine epidemic prevention and safety management personnel, the establishment of Chinese herbal medicine epidemic prevention and management system.
Registration of overseas enterprises: The General Administration of Customs, based on the results of risk analysis, determines the directory of medicinal materials species that need to be registered overseas and implements dynamic adjustment. The General Administration of Customs to export to China need to implement overseas registration of medicinal varieties of overseas production enterprises to implement registration, registration is valid for four years.
3. Quarantine Approval
Imported Chinese herbal medicines need to apply for imported animal and plant quarantine approval, the owner of the goods or his agent shall, before signing the trade contract, obtain the “People's Republic of China Imported Animal and Plant Quarantine License” in accordance with the “Measures for the Administration of Imported Animal and Plant Quarantine Approval”.
4、On-site Supervision
Documentary Audit - Customs accepts the declaration of the enterprise and audits whether the information submitted by the enterprise conforms to the requirements of laws, administrative regulations and administrative regulations on declaration of imported goods by the Customs.
Cargo Inspection - Customs implement port inspection of imported Chinese herbal medicines according to the instructions of the business system, mainly including:
(i) Inquire about the departure time and port of shipment, the country or region of transit, and the loading list;
(ii) whether the packaging is intact, whether with animal and plant packaging, bedding materials, in line with the provisions of the supervision and management of packaging and quarantine measures;
(C) whether the Chinese herbal medicines have any corruption and deterioration phenomenon, whether it carries harmful organisms, animal excreta or other animal tissues, etc., whether it carries animal carcasses, soil and other prohibited substances.
5、Laboratory testing
On-site quarantine found in pests, disease and insect infestation symptoms, or according to the relevant procedures for laboratory quarantine, the Customs and Excise Department shall sample the imported Chinese herbal medicines and send them to the laboratory.
6, designated places for storage, processing
Chinese herbal medicines before obtaining the certificate of quarantine shall be stored in the Customs approved location, without the permission of the Customs, any unit or individual shall not be transferred, sales, processing.
7、Quarantine results assessment
Imported Chinese herbal medicines by the quarantine qualified, the Customs issued “proof of entry inspection and quarantine of goods” before the sale, use or storage in designated enterprises, processing.
Quarantine failed, the Customs and Excise Department to issue a notice of quarantine treatment, by the owner or his agent under the supervision of the Customs and Excise Department, for the decontamination, return or destruction of the treatment, by the decontamination treatment is allowed to enter the country.
8、Quarantine treatment of means of transportation
Shipment of imported Chinese herbal medicine means of transportation and containers should meet the safety and health requirements. The need to implement anti-vaccination disinfection treatment should be implemented under the supervision of the Customs and Excise Department at the port of entry anti-vaccination disinfection treatment. Without the permission of the Customs, shall not be unloaded from the means of transport, container or delivery of imported Chinese herbal medicines.






